Coxsackie – Doing Damage to Our Very Core: The Energy Delivery Mechanisms of our Heart?
Published on 8 September, 2023
Coxsackie (named after where it was first identified, in Coxsackie, New York) is an enterovirus that belongs to the family of Picornaviruses. These are small positive-strand RNA viruses without a lipid membrane. It falls under the same genera as polio, echovirus, and other enteroviruses. Its transmission is oro-faecal, and its site of primary infection is the gut.
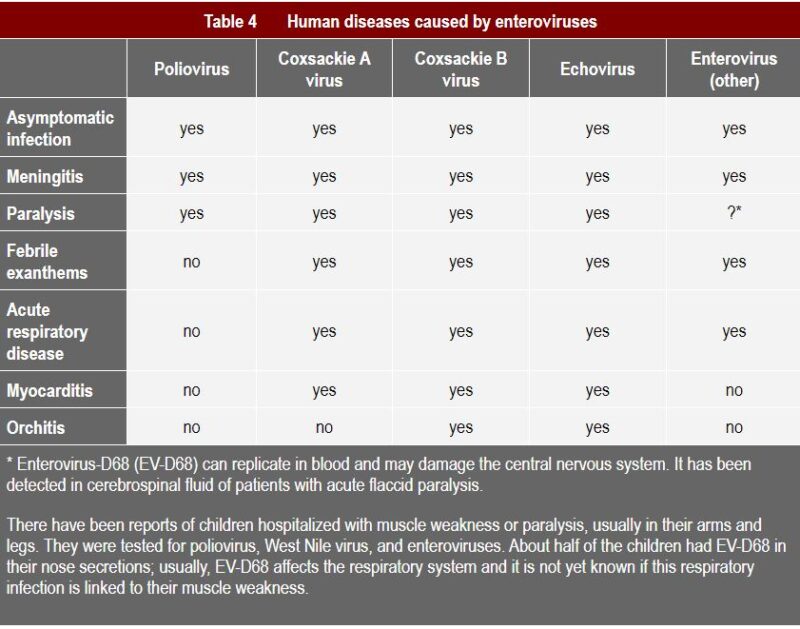
Coxsackie A virus is usually associated with fever, surface rashes, and can cause herpangina, as well as hand, foot, and mouth disease (1). Type B typically causes internal symptoms: it can trigger illness ranging from gastrointestinal distress to full-fledged pericarditis and myocarditis (Coxsackievirus-induced cardiomyopathy) (2). Subtypes of both A and B can cause very severe symptoms, as can be seen from the table below, such as meningitis and even paralysis.
Coxsackie B virus is very common in the UK, in the experience of Dr. Armin Schwarzbach of Arminlabs who says:
“The level of infection in the UK that we are picking up in our testing exceeds that of any other country, by percentage of positive titres in those tested – especially IgA, suggesting current infection of the mucosal membranes. It is unclear why that is – we urgently need studies.”
Coxsackie virus B can trigger an autoimmune attack on translocator protein, also called the adenine nucleotide translocator (ANT), in the heart (3). This appears to be because its VP (viral protein) capsid protein is cross-reactive to mitochondrial translocator protein (4).
The ANT is the only transport system for ADP and ATP – it is the most important link of the body’s energy producing and consuming processes. Autoantibodies against the adenine nucleotide transporter were found in the sera of patients suffering from myocarditis and dilated cardiomyopathy that were capable of inhibiting nucleotide exchange activity (5).
The mRNA pattern was found to be equivalent to the ANT isoform protein distribution in mitochondria (6). The importance of the virus in this process was shown by the fact that virus-positive patients had an approx. four times higher risk of isoform pattern alteration than those without enterovirus infection (7).
This is a huge discovery, as mitochondria – the energy powerhouses in our cells –– are rich in these translocator proteins (ANT)
Cross-reactivity between the ANT protein and the calcium channel was also observed (8).
ANT antibodies bind specifically at the calcium channel of the cell surface and change calcium influx into the cell (9). Raised intracellular calcium concentration causes intramitochondrial calcium overload and lowers mitochondrial transmembrane potential (10). This appears to be a frequent finding, too, in Acumen tests of patients with ME-type symptoms, and recent research from Australia is also finding calcium channelopathies in the NK cells of ME/CFS patients (11).
Could it be that they are suffering from blocked/lowered mitochondrial membrane potential -– at least in some cases – due to Coxsackie B?
The group of doctors and scientists around Professor Malcolm Hooper (The John Richardson Research Group, including Dr. Irving Spurr, sadly recently deceased) have long contended that Coxsackie B underlies many cases of so-called ME/CFS.
The ME/CFS researcher Professor Peter Behan suggested that the ANT autoantibody might be the cause of many symptoms of the condition as far back as the 80’s. One guinea pig study found that the measured energy output from hearts impacted by the ANT autoantibody was five times less than the energy output from healthy controls, and the hearts of the former animals produced more than twice the amount of lactate (12). The 2009 energy metabolism studies of Dr. Sarah Myhill, Professor Norman Booth (†) and Dr. John McLaren-Howard also found that:
ME/CFS patients have major disruption of their ANT protein – in both transport of ATP from the mitochondria into the cytosol and ADP from the cytosol into the mitochondria (13).
Blocking of the translocator proteins is often found in Acumen tests by heavy metals, pesticides, and other contaminants: that they have been disabled by the Coxsackie B virus certainly also appears another possibility in light of these studies.

A 2010 mouse study found that in Coxsackie B myocarditis, a reduction in IL-17 reduced ANT autoantibodies (14). Th17 cells produce IL-17 and have been associated with the promotion of viral replication. N-acetyl-glucosamine has been shown to reduce Th17 and IL-17, as can several other agents. Interestingly, the article that found this is entitled “N-acetylglucosamine inhibits T-helper 1 (Th1)/T– helper 17 (Th17) cell responses and treats experimental autoimmune encephalomyelitis”.
This would appear to bring us round full circle to the PANS/PANDAS pathology that many specialists have said should really be renamed autoimmune encephalitis. Perhaps the underlying drivers of these merciless diseases are not so different after all.
You can view this article and others in our October 2018 Newsletter.
You can learn more about Coxsackie Virus and the tests available fro AONM and ArminLabs HERE.
Please see our Mitochondrial Testing Page to see what Mitochondrial tests are available.
Don’t forget AONM now have an online SHOP where you will find some specially chosen products and some of the vast array of ArminLabs tests that we offer.
The ArminLabs Order Form with a comprehensive list of tests available is HERE.
If you would like us to contact you with more information about testing please fill in your details on THIS PAGE and one of our advisors will get back to you.
If you would like to subscribe to our newsletter and mailing list for any promotional offers and events such as live webinars and training for Practitioners please send us your details via our Contact Page.
References:
1. https://www.uptodate.com/contents/hand-foot-and-mouthdisease-and-herpangina
2. https://www.medicinenet.com/coxsackie_virus/article.htm
3. Schultheiss HP, Schulze K, Dorner A: Significance of the adenine nucleotide translocator in the pathogenesis of viral heart disease. Mol Cell Biochem. 1996, 163-164: 319-327
4. Ibid
5. K Schulze, B F Becker, R Schauer and H P Schultheiss. Antibodies to ADP-ATP carrier–an autoantigen in myocarditis and dilated cardiomyopathy–impair cardiac function. Circulation. 1990;81:959-969
6. The Role of Immune Mechanisms in Cardiovascular Disease, edited by Heinz-Peter Schultheiss, Peter Schwimmbeck, 1996, Springer
7. Dörner A, et al. The shift in the myocardial adenine nucleotide translocator isoform expression pattern is associated with an enteroviral infection in the absence of an active T-cell dependent immune response in human inflammatory heart disease. J Am Coll Cardiol. 2000 Jun;35(7):1778-84
8. Kühl U et al..Antibody-mediated enhancement of calcium permeability in cardiac myocytes. J Exp Med. 1988 Dec 1;168(6):2105-19; Davies W et al. Antibodies against ADPATP carrier enhance Ca2+ current in isolated cardiac myocytes. Am J Physiol. 1988 Oct;255(4 Pt 2):H960-4
9. Ibid.
10. Miyamoto S et al. Ca2+ dysregulation induces mitochondrial depolarization and apoptosis: role of Na+/Ca2+ exchanger and AKT. J Biol Chem. 2005 Nov 18;280(46):38505
11. Nguyen T et al. Impaired calcium mobilization in natural killer cells from chronic fatigue syndrome/myalgic encephalomyelitis patients is associated with transient receptor potential melastatin 3 ion channels. Clin Exp Immunol. 2017 Feb;187(2):284-293
12. Op. cit. Circulation. 1990;81:959-969
13. Myhill S, Booth NE, McLaren-Howard J. Targeting mitochondrial dysfunction in the treatment of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) – a clinical audit. Int J Clin Exp Med. 2013;6(1):1-15
14. Yuan J et al. Neutralization of IL-17 inhibits the production of anti-ANT autoantibodies in CVB3-induced acute viral myocarditis. Int Immunopharmacol. 2010 Mar;10(3):272-6.